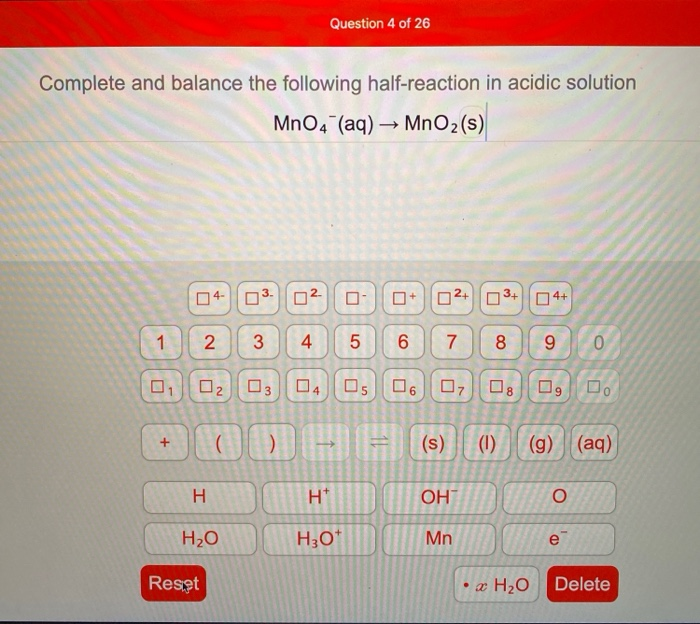
Complete and Balance the Following Half-reaction in Acidic Solution Mno4-
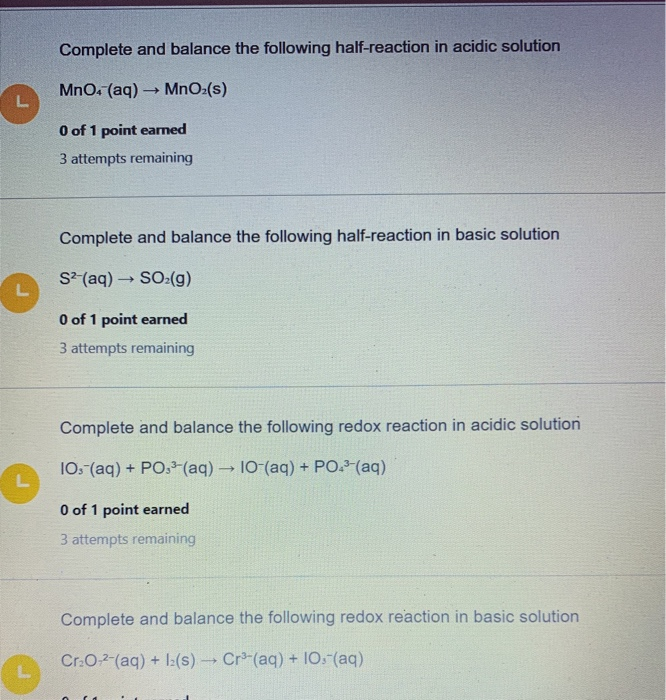
Complete and balance the following half-reaction in acidic solution MnO4 aq MnO2 s. SO3 2- _ SO4 2- _.

Unsaved Change Moving To Another Question Will Save This Response In 2022 No Response Change Moving
Chemistry questions and answers.

. Sign In to Writing Essays Science. Balance O by adding H₂O to the deficient side. Here is a second half-reaction.
Separate the equation into two half-reactions. The number of atoms of each element is the same on each side of the equation. Here is the half-reaction to be considered.
Use the lowest possible coefficients. Click hereto get an answer to your question UN Complete and balance the following equation. Use H to balance H.
At the end you use OH to convert to base. 3Sb3aq BrO3-aq 6Haq3Sb5aq Br-aq 3H2Ol Explanation. Start your trial now.
MnO4 - _ MnO2 _. When we want to balance redox reaction equations we must ensure that the number of electrons lost in the oxidation half reaction equation is equal to the number of electrons gained in the reduction half reaction equation. Which statement best describes how chemical equations demonstrate conservation of mass.
Chemistry questions and answers. Identify Oxidation and Reduction half Reaction. The number of reactants is the same as the number of produc.
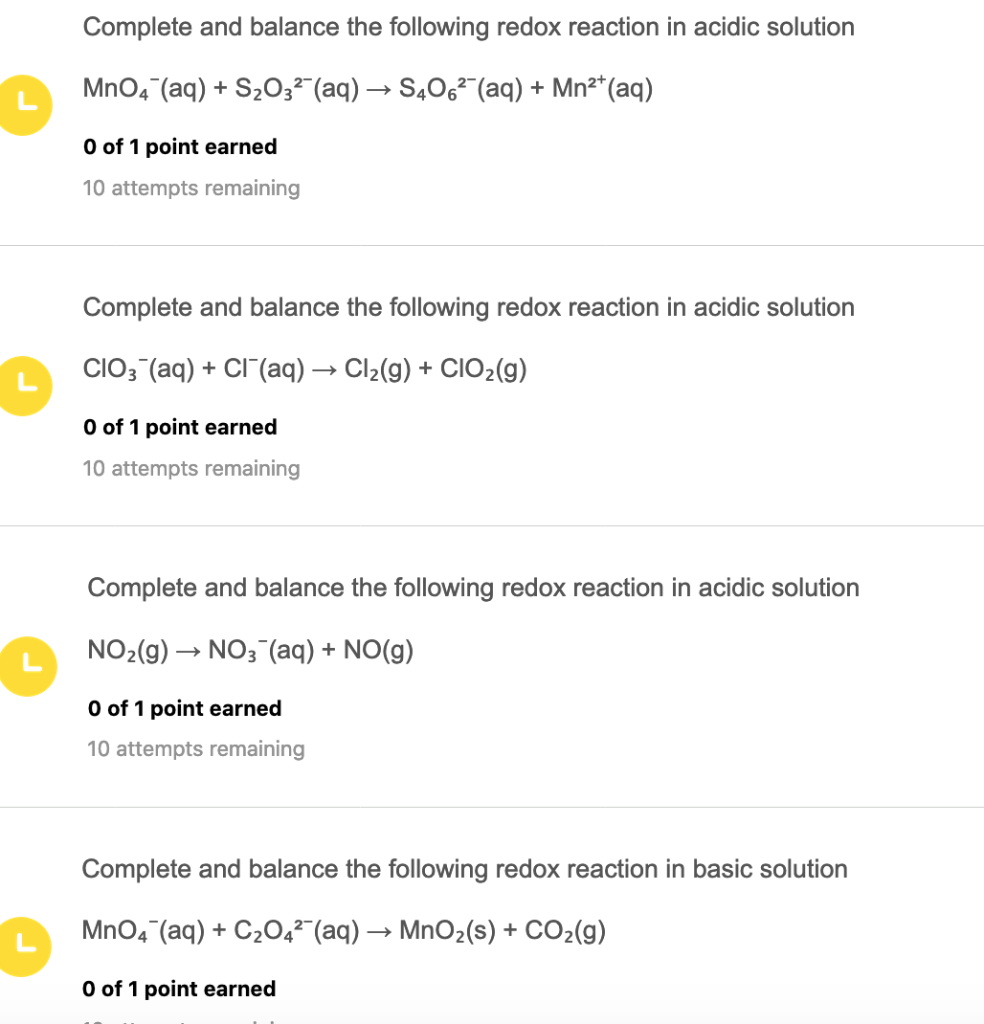
4MnO4- aq 5CH3OH aq 11H2O l 12H aq 4Mn2 aq 5HCO2H aq can someone please help. Balancing a redox reaction in basic aqueous solution in ten easy steps. The unbalanced redox equation is as follows.
Separate into Half Equations. How do I balance the following skeleton redox equation for a reaction occurring in acidic solution. One more question- how do I balance this half-reaction in terms of atoms.
Sign In to Tutor. The change in the oxidation number per Mn atom is 5. MnO 4 SO 2 SO 42 Mn 2acidic All atoms other than H and O are balanced.
Balance the following equation. Complete and balance the following redox reaction in acidic solution H2O2aq Cr2O2-aq O2g A. The oxidation number of Mn changes from 7 to 2.
MnO₄ ----- MnO₂ Reduction I -----I₂ Oxidation Step3. MnO 4 --- Mn 2 It is to be balanced in acidic solution. Redox reactions are those in which one of the molecules is.
M nO 4 8H 5e M n2 4H 2Ol ii For each half-equation charge and mass are balanced ABSOLUTELY and thus it reflects stoichiometry. Complete and balance the following half-reaction in acidic solution S2O32- aq S4062- aq Complete and balance the following half-reaction in basic solution N2 g NH3 g Complete and balance the following redox reaction in acidic solution As2O3 s NO3 aq H3AsO4 aq N2O3 aq Complete and balance the. The compounds are the same on each side of the reaction.
Balance the following half reaction in acid solution. First Write the Given Redox Reaction. The balanced equation for the reaction in acidic solution is 14 H aq 2 Mn2 aq 5 NaBiO3 s 2 MnO4 aq 5 Bi3 aq 5 Na aq 7 H2O l There are 7 water molecules on the product side.
SO3 2- H2O _ SO4 2- _. Chemistry questions and answers. TO produce a balanced equation we adds i and ii in such a way as to remove the.
Complete and balance the following half-reactions. ChemistryQA LibraryComplete and balance the following half-reaction in acidic solution MnO4-aq MNO2s Complete and balance the following half-reaction in acidic solution MnO4-aq MNO2s close. Include states-of-matter under the given conditions in your answer Br aq MnO4aq chem again.
MnO4 H2S Mn2 S acidic medium. In a particular redox reaction MnO2 is oxidized to MnO4 and Cu2 is reduced to Cu. Find step-by-step Chemistry solutions and your answer to the following textbook question.
MnO4 - _ MnO2 2 H2O _. Complete and balance the following half-reaction in acidic solution MnO4 aq MnO2 s Complete and balance the following half-reaction in basic solution 103- aq 10- aq. In each case indicate whether the half-reaction is an oxidation or a reduction.
Use water to balance O. The oxidation number of S changes from 4 to 6. Cr 2 O 7 2 --- Cr 3 acidic soln As I go through the steps below using the first half-reaction try and balance the second half-reaction as you go from step to step.
The change in the oxidation number is 2. MnO4Mn2aq MnO4Mn23e MnO48H5eMn24H2O MnO48HMn24H2O MnO48HMn24H2O5e none of the above. Balance H by adding H to the deficient side.
Complete and balance the following half-reaction in acidic solution MnO4 aq MnOz s 0 of 1 point earned 3 attempts remaining Complete and balance the following half-reaction in basic solution S2 aq SO2 g O of 1 point earned 3 attempts remaining Complete and balance the following redox reaction in acidic solution 103-. HNO2 MnO4- yield to Mn2 NO3- Chemistry. In basic solution you balance redox equations as if they were in acid.
Balance the atoms undergoing change in the Oxidation number. Question 3 of 16 Complete and balance the following half-reaction in acidic solution MnO4 aq MnO2 s. MnO4 - 4 H _ MnO2 2 H2O _.
Balance charge by adding electrons to the more. Sign In to Solutions. The state of matter is the same on each.
Balance the following oxidation-reduction reactions which occur in acidic solution using the half-reaction method. Operatorname Sn 2 a q longrightarrow operatorname Sn 4 a q acidic solution. MnO₄ I ----- MnO₂ I₂.
Complete and balance the following equation. MnO4 aq CH3OH aq Mn2 aq HCO2H aq acidic solution I KNOW ITS a redox so i followed the steps and got. Complete and balance the equation for this reaction in acidic solution.
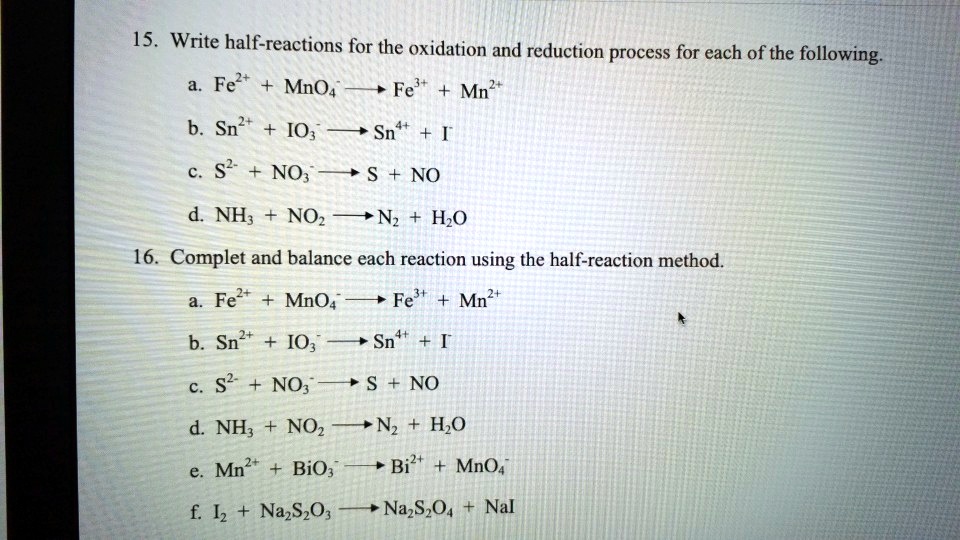
Solved 15 Write Half Reactions For The Oxidation And Reduction Process For Each Of The Following Fel Mno4 Fe Mn2 B Sn2 Io Sn S2 No No Nh Noz Nz Hzo 16 Complet
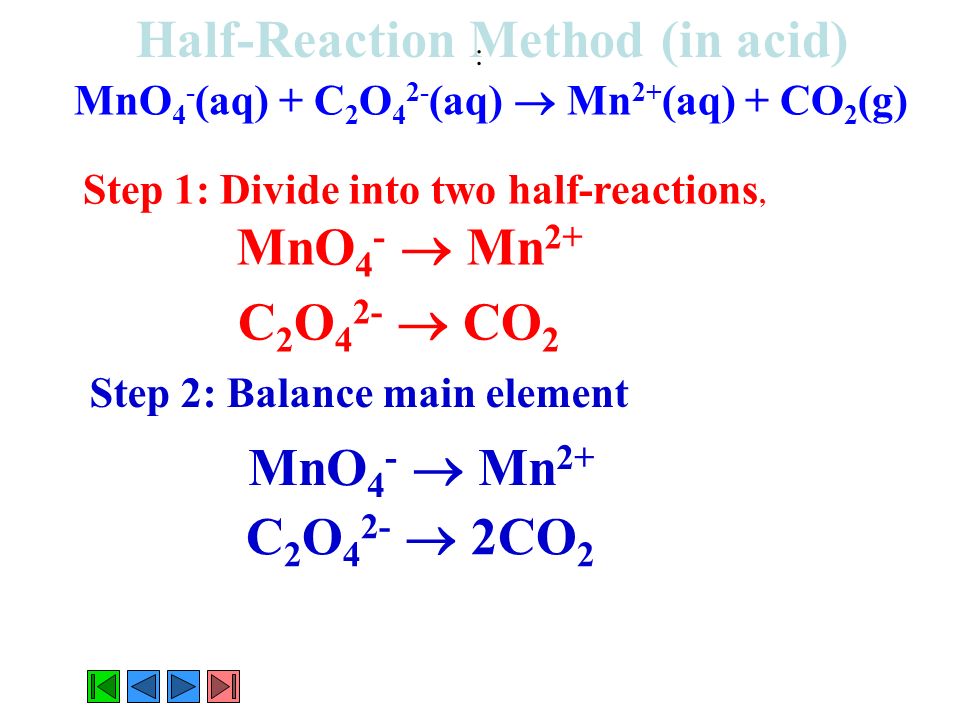
Half Reaction Method For Balancing In An Acid Or Base Ppt Download

Mno 4 Aq Al S Mn2 Aq Al3 Aq In 2022 Redox Reactions Reactions Solutions
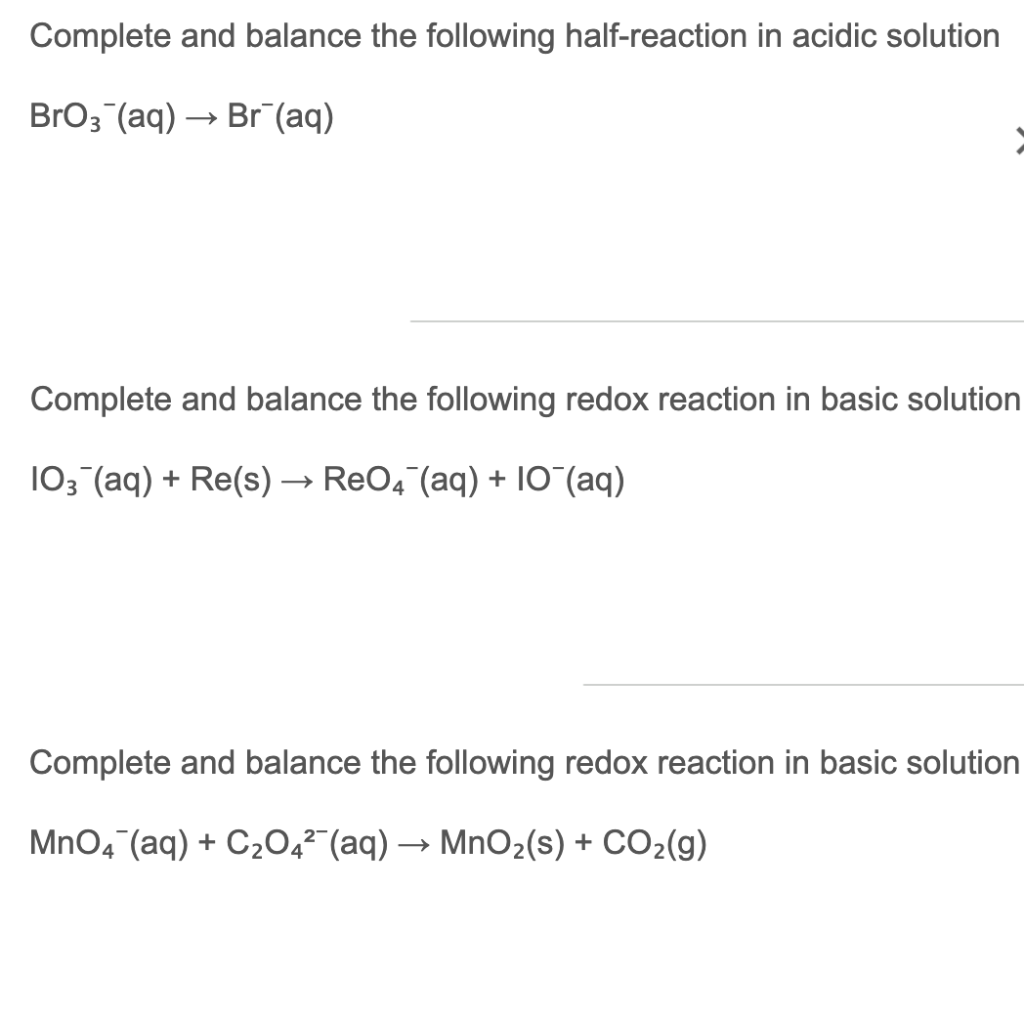
Solved Complete And Balance The Following Half Reaction In Chegg Com

Solved Complete And Balance The Following Redox Reaction In Chegg Com
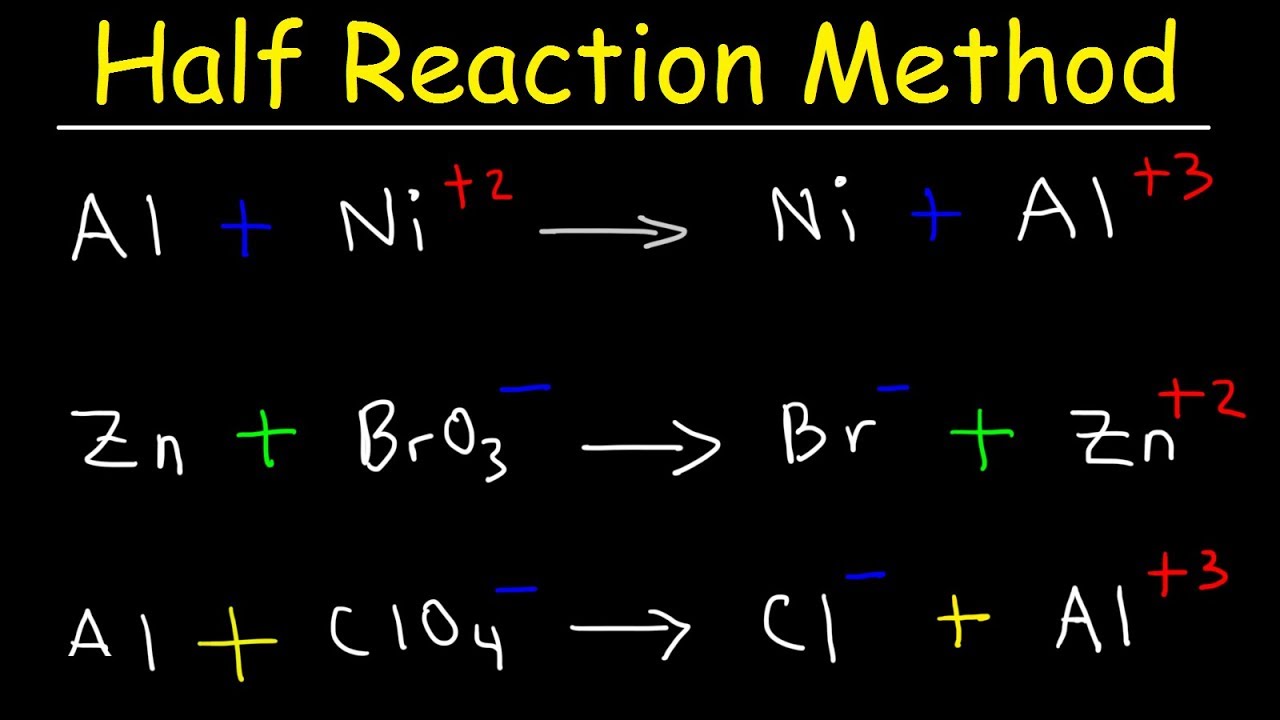
Half Reaction Method Balancing Redox Reactions In Basic Acidic Solution Chemistry Youtube

Balancing Redox Reaction By Ion Electron Method Kmno4 And Sncl2 Redox B Redox Reactions Electrons Chemistry
Solved Complete And Balance The Following Half Reaction In Chegg Com
Solved Question 4 Of 26 Complete And Balance The Following Chegg Com

Balance The Redox Reaction By Ion Electron Method Or Half Reaction Method Mno4 So3 2 Mno2 So4 2 Youtube

Balance The Redox Reaction By Ion Electron Method Half Reaction Method Ch3oh Mno4 Co3 2 Mno4 2 Youtube
Solved Complete And Balance The Following Redox Reaction In Chegg Com

Solved Complete And Balance The Following Half Reaction In Chegg Com

Balance The Redox Reaction For Mno4 H2so3 Mn2 Hso4 Youtube

Solved Complete And Balance The Following Redox Reaction In Chegg Com
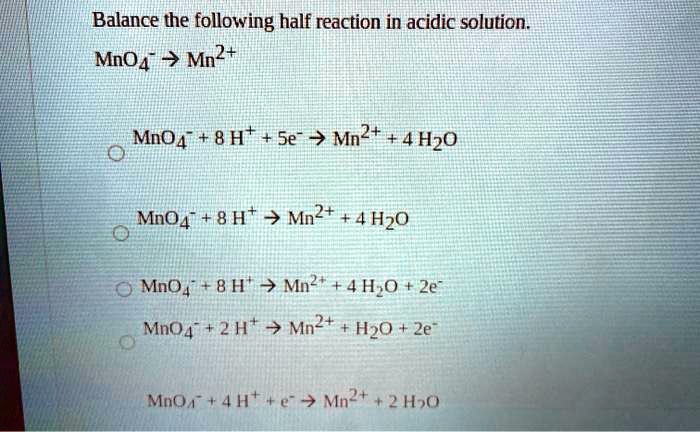
Solved Balance The Following Half Reaction In Acidic Solution Mno4 Mn2 Mno4 8 H Se Mn2 4 H20 Mno4 8 Ht 7 Mn2 4 H2o Mno4 8 H 7 Mnz 4h 0

Mno4 I Mno2 Io3 Balance The Redox Reaction By Ion Electron Method Or Half Reaction Method Youtube
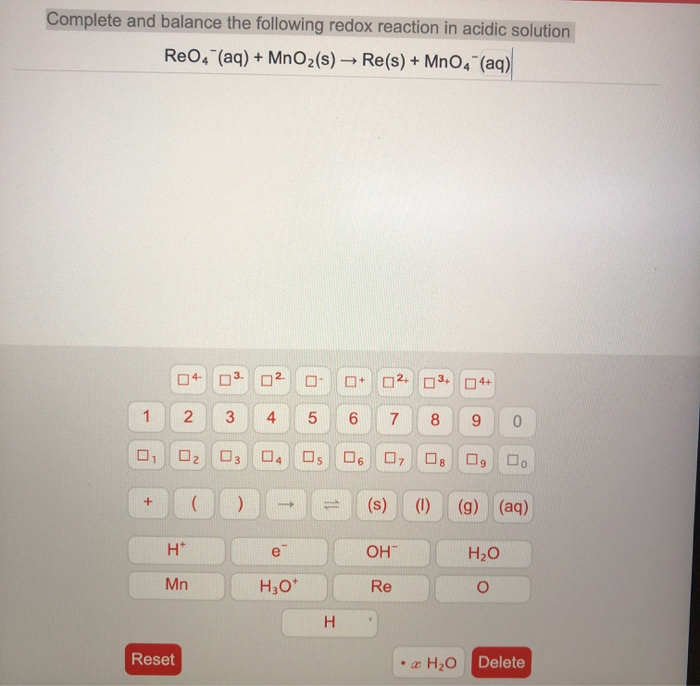
Solved Complete And Balance The Following Redox Reaction In Chegg Com

Pin By Saitech Informatics On Balancing Of Chemical Reaction Chemistry Worksheets Equations Redox Reactions
Comments
Post a Comment